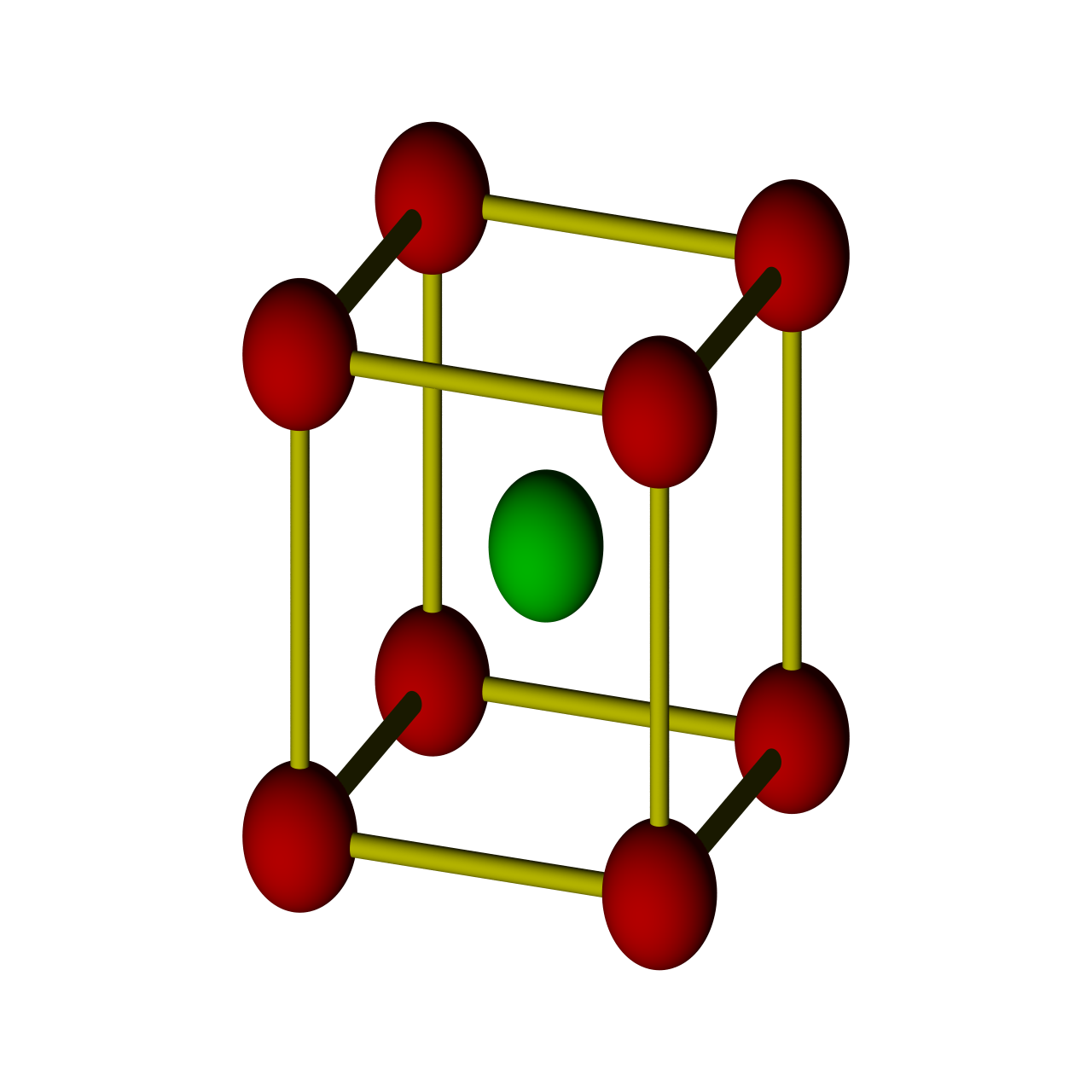
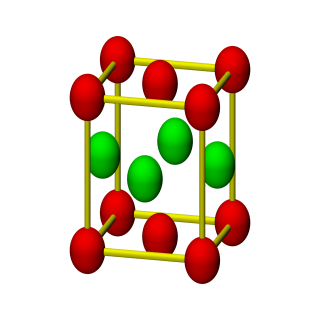
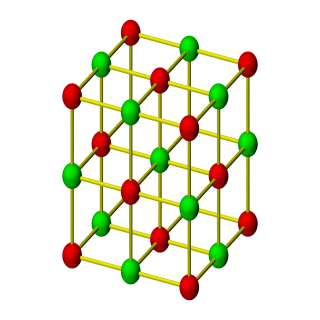
3D Nanoparticle Self-Assembly
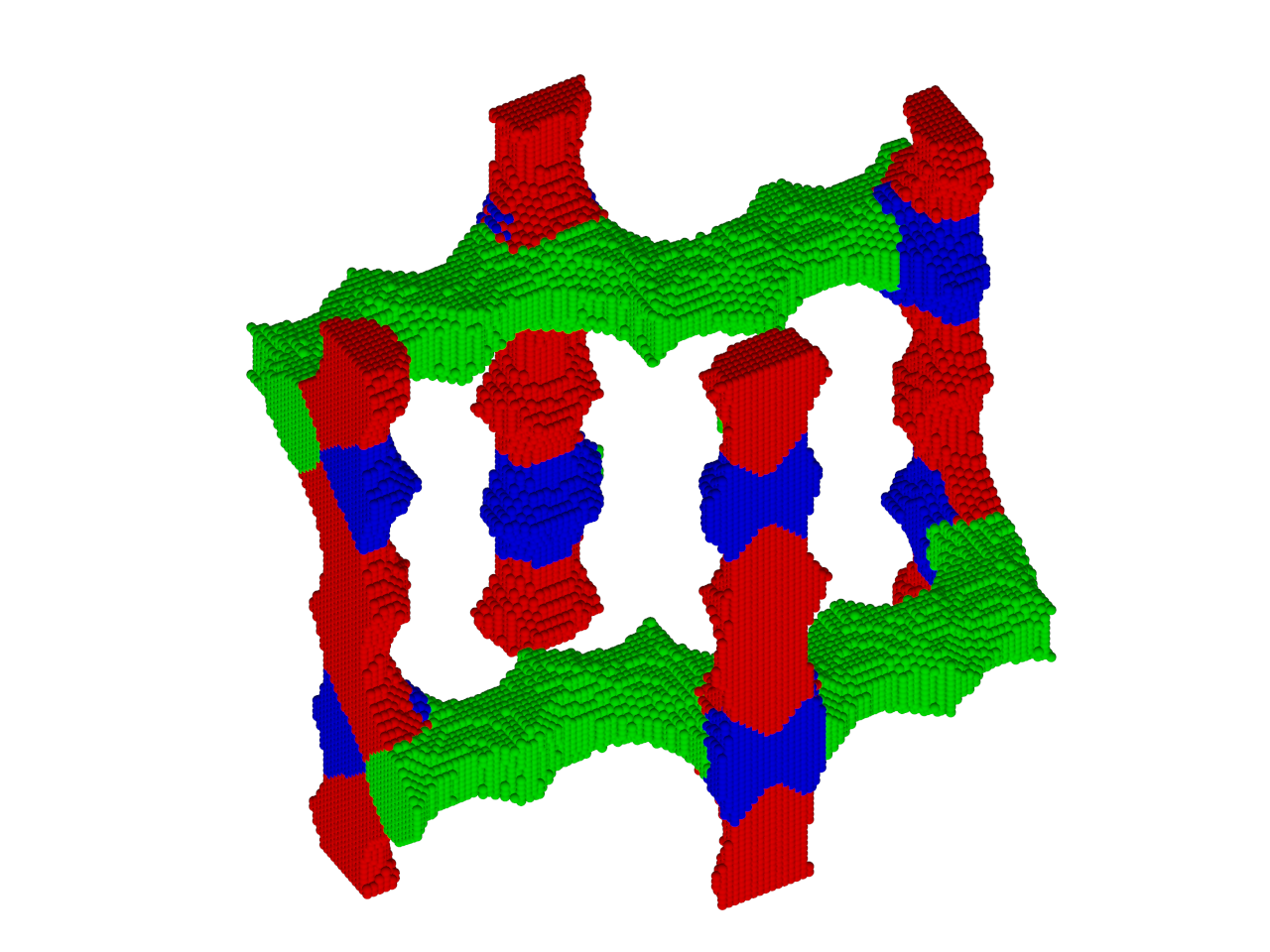
When two solutions containing electrically stabilized nanoparticle suspensions but oppositely charged are mixed together, they form aggregates and precipitate out of the solution. Under carefully controlled conditions, these aggregates are crystalline in nature and also substitutionally ordered. Depending upon their structure, these crystalline materials posses novel properties that can find applications in diverse areas such as nanoelectronics, plasmonics, photonics, catalysis, high-density data storage, etc.
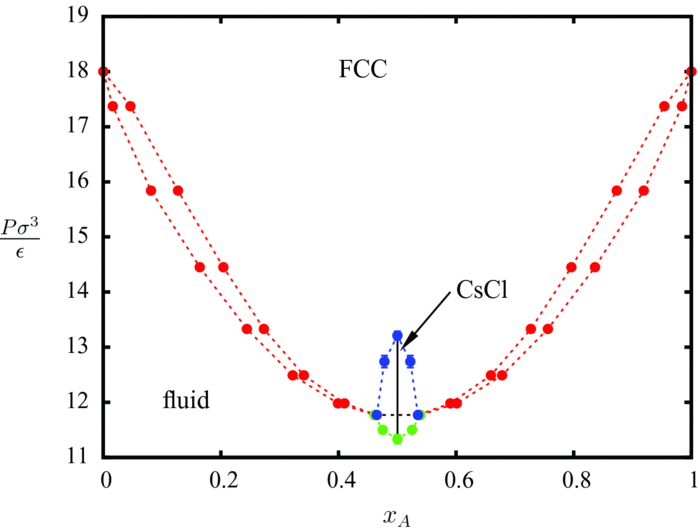
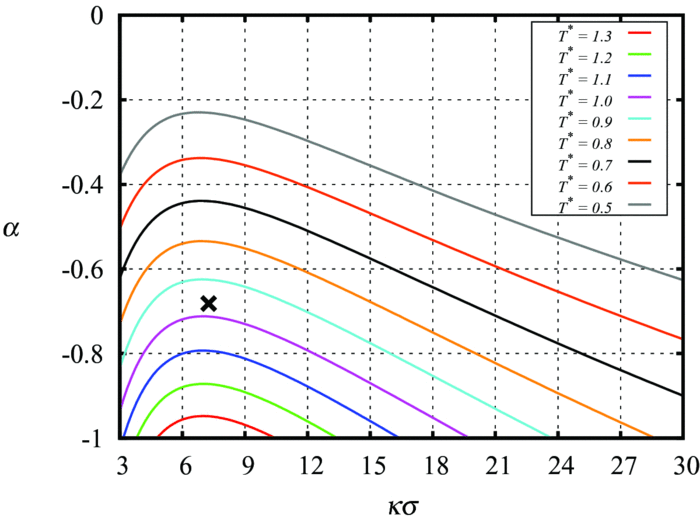
This process of 3D self-assembly can be viewes as equivalent to crystallization from a melt or solution. Thus the struture of the self-assembled material is dictated to a large extent by its free-energy. In this project, we computed the free-energies of various crystalline aggregates from Monte-Carlo molecular simulations and in turn computed solid-fluid phase diagrams for various mixtures of oppositely charged nanoparticles. From this study, we were able to construct a map of inter-particle interactions with the final structure of the self-assembled material.
To know more about our work on 3D nanoparticle self-assembly, please read our following papers.
- Sudeep N. Punnathanam, J. Chem. Phys., 139, 086101 (2013)
- Ganeshprasad Pavaskar and Sudeep N. Punnathanam, J. Chem. Phys., 138, 174504 (2013)
- Ganeshprasad Pavaskar, Siddharth Sharma and Sudeep N. Punnathanam, J. Chem. Phys., 136, 134506 (2012)
Liquid-Phase Adsorption in Zeolites and MOFs
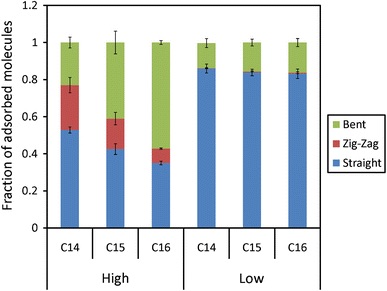
Many industrial adsorption processes occur under liquid-phase conditions. For example, the Sorbex processes are run in the liquid phase for separating normal paraffins from branched paraffins and cycloparaffins (Molex process), olefins from olefin-paraffin mixtures (Olex process), p-xylene from other C8 hydrocarbons (Parex process), p-diethylbenzene from mixed diethylbenzene isomers (p-DED), and fructose from corn syrup (Sarex). Under these conditions, adsorbent pores are full of molecules and packing of adsorbate molecules, in addition to adsorbate-adsorbent interactions, play an important role in determining selectivity.
Due to high adsorbate density inside the pores of adsorbent, study of liquid-phase adsorption using molecular simulations is very challenging and special techniques such as configurational-bias Monte Carlo (CBMC) and parallel-tempering are required. We have developed and applied these methods to study adsorption of long-chain alkanes in zeolties LTA-5A and silicalite, as well as xylene separation in MOF-5.
To know more about our work on liquid phase adsorption please read our following papers.
- Hari S. Ganesh and Sudeep N. Punnathanam, Adsorption, 20, 729-736 (2014)
- Sudeep Punnathanam, Joeri F. M. Denayer, Inge Daems, Gino V. Baron and Randall Q. Snurr J. Phys. Chem. C, 115, 762-769 (2011)
Methane Storage and CO2 Capture in Functionalized Porous Carbons
Development of an alternative, renewable, carbon-free, and sustainable energy source is one of the major challenges in the 21st century. In this regard, natural gas (NG) has emerged as a viable transportation fuel in many parts of the world. However, replacement of gasoline with natural gas requires development of technologies for on-board storage. In this regard Adsorbed Natural Gas (ANG) is emerging as promising technology with activated carbons as the adsorbent material
Flue gas emissions from coal based power plants account for the largest sources of carbon dioxide emissions today. Since carbon dioxide is one of the primary greenhouse gases that contributes to global warming and climate change, carbon capture from flue gases remains an outstanding challenge in the effort to contain global warming. Selective adsorption of CO2 from a flue gas mixture is emerging as a promising alternative to absroption in amine-based solutions.
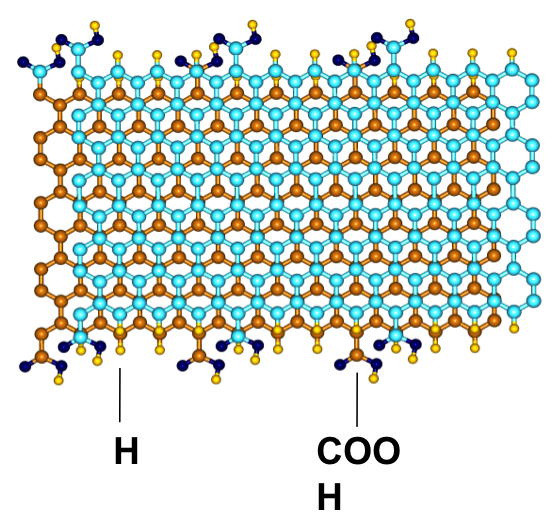
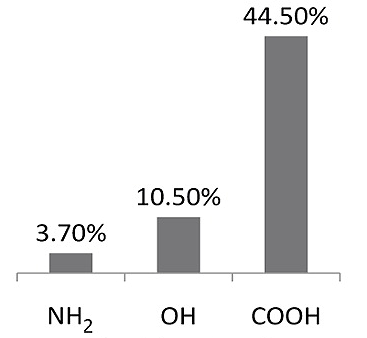
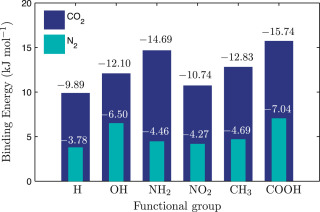
Towards these ends, we have performed a combination of ab initio and classical Monte Carlo simulations to assess the role of organic functional groups present in porous carbons towards enhancement of Methane storage as well as CO2 capture from flue gases. To know more about our work please read the following papers.
- Tonnishtha Dasgupta, Sudeep N. Punnathanam and K. Ganapathy Ayappa Chem. Eng. Sci, 121, 279-291 (2015)
- Vinay Kandagal, Amardeep Pathak, K. Ganapathy Ayappa and Sudeep N. Punnathanam J. Phys. Chem. C, 116, 23394-23403 (2012)
- Brandon C. Wood, Shreyas Y. Bhide, Debosruti Dutta, Vinay Kandagal, Amardeep Pathak, Sudeep N. Punnathanam, K. Ganapathy Ayappa and Shobana Narasimhan J. Chem. Phys., 137, 054702 (2012)