Clathrate Hydrates
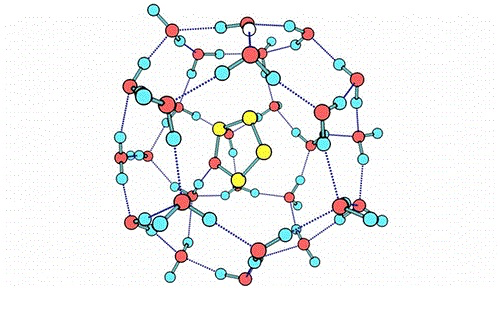
Gas hydrates or clathrate hydrates are crystalline inclusion compounds of water and light gases such as methane, ethane, carbon dioxide, etc. They are formed under conditions of low temperature and high pressure, e.g., at 273 K, Methane hydrates form at a pressures greater than 10 MPa. Gas hydrates, especially those containing methane are naturally occuring in deep sea floors and in permafrost regions. They also form in oil and natural gas transmission pipelines which often lead to their blockage.
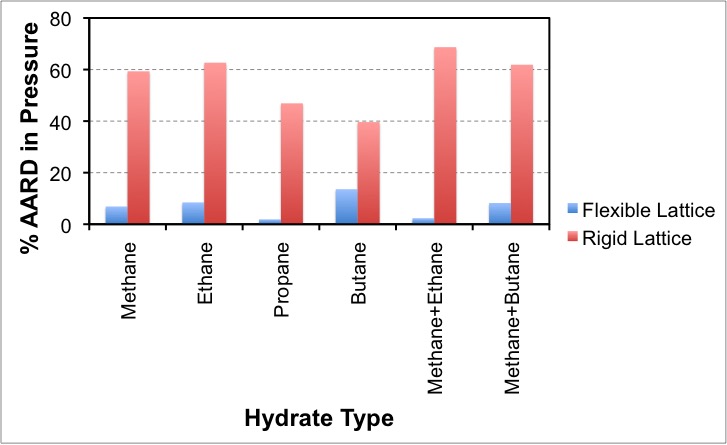
In our lab, we study clathrate hydrates using molecular simulations such as Monte Carlo and molecular dynamics. Recently, through simulations, we have developed a method to include the contribution of flexibility of the water-lattice to the partition function of clathrate hydrates. This work has helped us to considerably improve the accuracy, robustness and thermodynamic consistency of theory for clathrate hydrates.
To know more about our work on clathrate hydrates, please read our following papers.
- Srikanth Ravipati and Sudeep N. Punnathanam, J. Phys. Chem. C, 119, 12365-12377 (2015)
- Srikanth Ravipati and Sudeep N. Punnathanam, Fluid Phase Equil., 376, 193-201 (2014)
- Srikanth Ravipati and Sudeep N. Punnathanam, J. Phys. Chem. C, 117, 18549-18555 (2013)
- Srikanth Ravipati and Sudeep N. Punnathanam, Ind. Eng. Chem. Res., 51, 9419-9426 (2012)
- Hrushikesh Pimpalgaonkar, Shivanand K. Vessam and Sudeep N. Punnathanam, J. Phys. Chem. B, 115, 10018-10026 (2011)
Crystal Nucleation
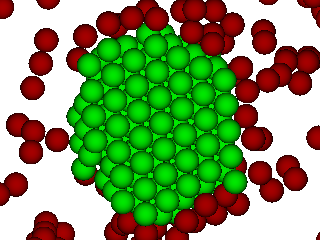
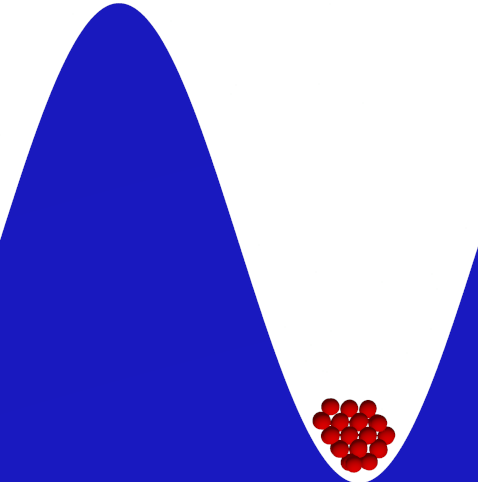
The primary mechanism of phase transition from fluid to solid is homogeneous crystal nucleation. Understanding the molecular mechanism of crystal nucleation has tremendous scientific and practical importance. One of the main challenges in designing a crystallization process is in controlling the crystal structure of the final product. The solid state of a given compound can exist is multiple crystalline structures, also known as polymorphs. This final crystalline structure depends on the conditions of crystallization such as pressure, temperature, solvent composition, presence of additives or impurities, nature of the external surface for crystallization, etc.
We simulate crystal nucleation in a variety of systems including binary hard-sphere mixtures, protein solutions and molecular crystals. The overall strategy in all these systems is to calculate the free-energy of formation of the critical nucleus inside the fluid phase. The techniques used for simulating crystal nucleation vary based on the nature of the surrounding fluid which can be a melt, a low-density vapor or a solution.
To know more about our work on crystal nucleation, please read our following papers.
- Praveen K. Bommineni and Sudeep N. Punnathanam, Faraday Discuss., 186, 187-197(2016)
- Srinivas R. Ganagalla and Sudeep N. Punnathanam, J. Chem. Phys., 138, 174503 (2013)
Electric Double Layer Capacitor
Supercapacitors such as alongwith rechargable batteries and dielectric capacitors are electrochemical devices that store energy. In terms of their performance, supercapacitors lie between batteries and capacitors. Compared to rechargable batteries, they have higher power densities but lower energy densities, where as compared to dielectric capacitors, they have higher energy densities but lower power densities.
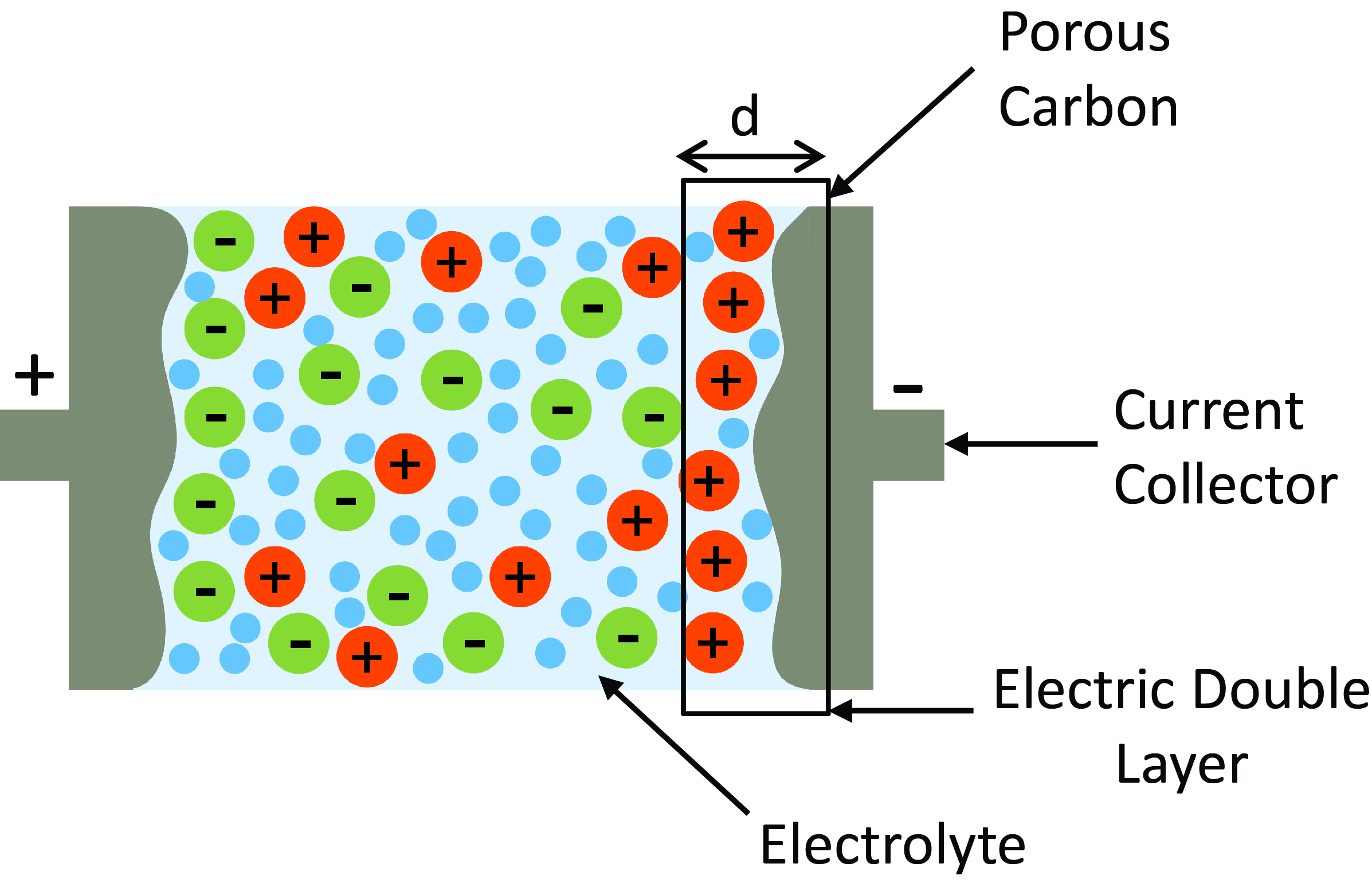
One such class of supercapacitors is an Electric Double Layer Capacitor (EDLC). A typical EDLC consists of two porous carbons that act as electrodes separated by an electrolyte solutions. Upon application of electric field, an electric double layer is formed along the pore walls of each electrode. Unlike dielectric capacitors, the energy here is stored due to the formation of these electric double layers. Continuum theories of double layer formation such as the Debye-Huckel theory or the Gouy-Chapman theory break down when the pore sizes of the electrodes become comparable to the size of the electrolyte ions. To overcome this problem, one has to resort to atomisitic simulations. In our group, we are actively developing techniques to atomistically simulate EDLCs.
To know more about our work on crystal nucleation, please read our following papers.
- Sudeep N. Punnathanam, J. Chem. Phys., 140, 174110 (2014)