 |
Development of Dengue vaccine tailored to infections in India
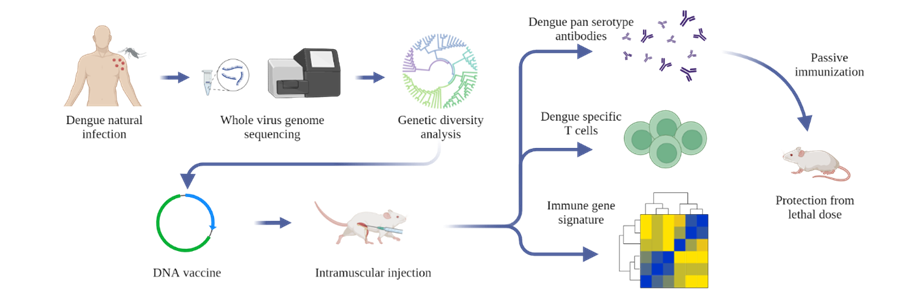 Dengue virus (DENV) is a mosquito-borne flavivirus with a single-stranded RNA genome that causes an estimated 390 million infections worldwide every year. All four serotypes of DENV (DENV1-4 sharing 65-70% sequence homology) are co-circulating in India simultaneously. Although more than a hundred thousand dengue cases are reported from India every year, the genomic diversity of the prevalent dengue viruses (DENVs) in the country is largely unknown. Using whole-genome sequencing of viral RNA from clinical human serum samples, Rahul Roy's group report 119 DENV full-genomes from four metropolitan sites across India from 2012 to 2018.
Dengue virus (DENV) is a mosquito-borne flavivirus with a single-stranded RNA genome that causes an estimated 390 million infections worldwide every year. All four serotypes of DENV (DENV1-4 sharing 65-70% sequence homology) are co-circulating in India simultaneously. Although more than a hundred thousand dengue cases are reported from India every year, the genomic diversity of the prevalent dengue viruses (DENVs) in the country is largely unknown. Using whole-genome sequencing of viral RNA from clinical human serum samples, Rahul Roy's group report 119 DENV full-genomes from four metropolitan sites across India from 2012 to 2018.
It has been known that homotypic secondary infection is generally asymptomatic, but heterotypic secondary infection is associated with increased chances of severe infection. The cross-reactive antibodies can enhance subsequent infection by a heterologous serotype (ADE: antibody-dependent enhancement). The envelope protein domain III (EDIII) has been identified as the major target of neutralizing and serotype-specific antibodies, while precursor membrane and EDI-II-directed antibodies are reported to enhance infection via ADE. Recent studies have found that EDIII-based DENV vaccines could circumvent ADE of infection in mice. Another target for the vaccine is NS1, which is a highly conserved protein among flaviviruses and has been shown to be evoking antibody and T cell responses previously. Team of researchers from NCBS, C-CAMP and IISc developed a DENV EDIII-based DNA vaccine candidate by integrating the consensus sequences from the circulating sequences of each serotype, incorporating the NS1 protein-coding region of DENV2. The vaccine generated robust antibody response and T cell response against all the four serotypes of DENV. Passive transfer of antibodies from the immunized mice also protected the non-immunized mice from the lethal dose of DENV infection. Source: https://doi.org/10.1016/j.ymthe.2022.01.013 |