 |
Particle nucleation
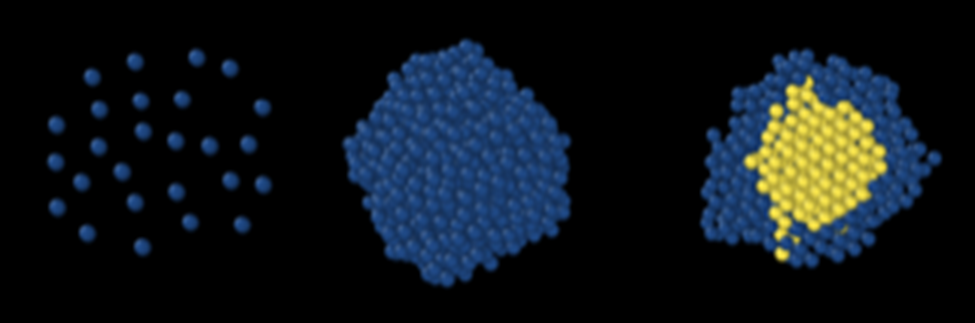 How crystals nucleate from dilute vapors and solutions? Although seemingly simple, the nucleation of crystals from the fluid phase is a complex phenomenon. Despite numerous computational and experimental studies, its mechanism is not completely understood. Recent studies have shown that, contrary to century-old classical nucleation theory, crystal nucleation follows a two-step mechanism where the molecules initially come together to form an aggregate, followed by crystal nucleation within the aggregate. In chemical process industries, an understanding of the nucleation mechanism is important for design and control of crystallization processes. For example, in the pharmaceutical industry, polymorph control (control of crystal structure) during crystallization is of vital technological and commercial importance. In such a scenario, Ravi Kumar Reddy Addula and Sudeep N Punnathanam from the Department of Chemical Engineering have developed a new molecular theory of crystal nucleation theory from dilute phases such as vapours and dilute solutions. The molecular theory with its basis in statistical mechanics, is able to provide the most complete description till date of the nucleation process and is expected to provide important insights into the mechanism of crystal nucleation from solutions. This should enable scientists and engineers to make improvements in process design and control of crystallization process. Ravi Kumar Reddy Addula and Sudeep N. Punnathanam, Molecular Theory of Nucleation from Dilute Phases: Formulation and Application to Lennard-Jones Vapor. Phys. Rev. Lett. 126, 146001 (2021) Source: https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.126.146001 |